Savings & Resources
Now Available through CVS Specialty Pharmacy!

Save through Siklos ® Savings Programs
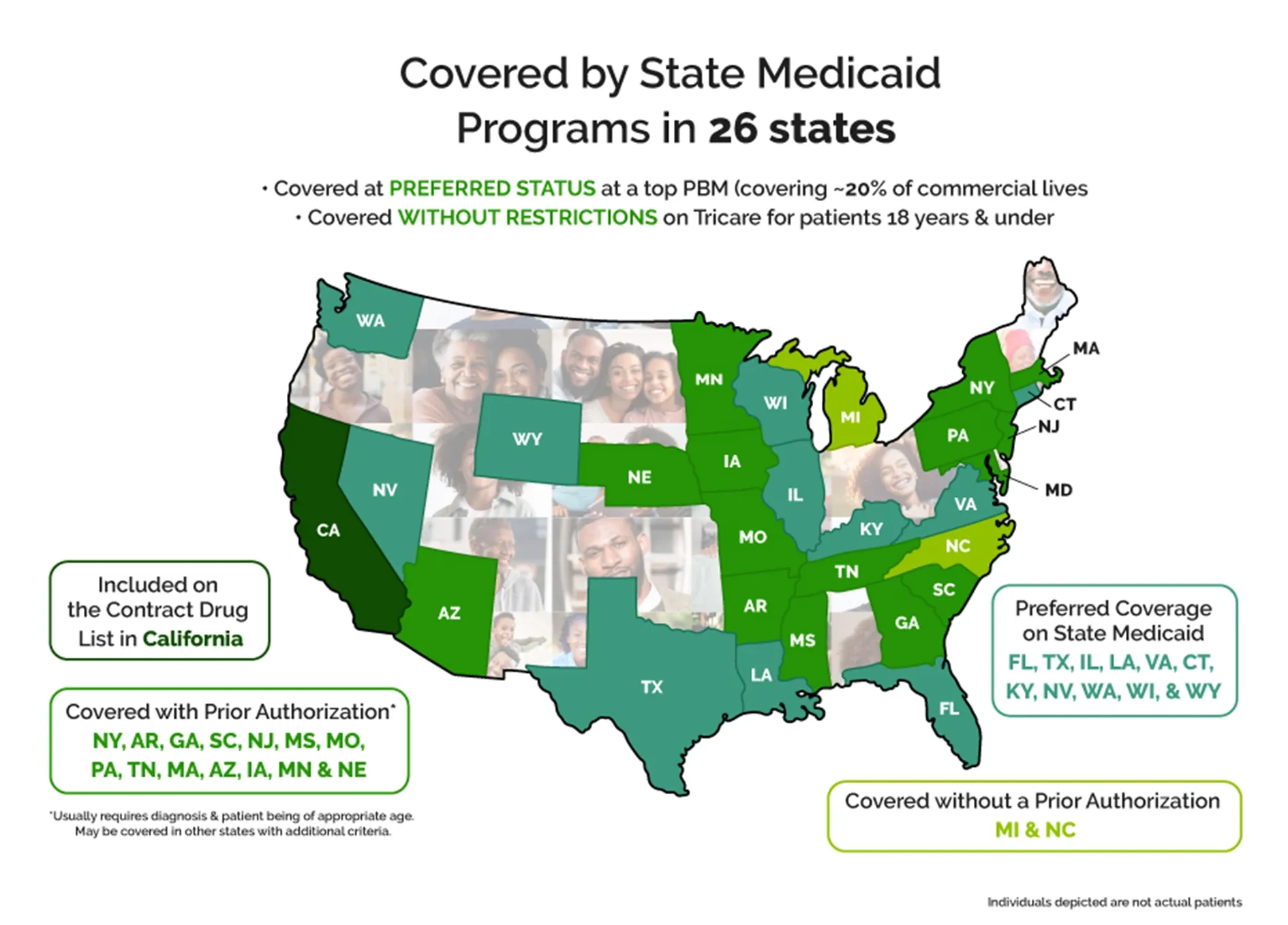
Siklos® is covered by many State Medicaid programs and now has Covered on State Medicaid in 22 states:
If you have commercial insurance, there are 2 easy ways to pay as little as $0* for you or your child’s Siklos® prescription!
Medunik USA also offers savings programs for patients that do not have commercial insurance or if their commercial insurance does not cover Siklos®.
Option 1: Fill Prescription at a Local Retail Pharmacy
Simply present an eligible Siklos® prescription at a participating pharmacy and your Siklos® eVoucher will be applied automatically – no coupon necessary.
If your pharmacy is not listed below and does not participate in the eVoucher program, that is ok! Just bring in a copy or show an electronic copy of the above Copay Card to your pharmacist.
Option 2: Mail Order Pharmacy
Ask your doctor to fax, call, or e-prescribe your or your child’s Siklos®prescription through the Siklos at Home® program. In addition to saving on the prescription costs, the Siklos at Home® program offers:
- Free home delivery (minimum purchase of $99)
- On-staff pharmacists to answer your product questions
- Assistance with insurance benefit verification
- Monthly refill reminder
Questions? Call (844) 716-HOME (4663)
If you do not have commercial insurance or your insurance does not cover Siklos®, Siklos at Home ® offers:
- Siklos® 100 mg tablets: pay as little as $99 for 60 tablets or $149 for 90 tablets*
- Siklos® 1,000 mg tablets: pay as little as $16.50/tablet*
*See Terms and Conditions
Siklos® Coverage
Patient Assistance Program – designed to help if affording a Siklos® prescription is difficult
Medunik USA believes that every patient with sickle cell disease has the right to access safe and effective medication. We understand that, at times, the cost of prescription medications can be a challenge. That is why we created our Patient Assistance Program.
If you cannot afford a Siklos® prescription, you or your child may be eligible to receive Siklos®at no charge.
To apply, please follow these steps:
- Download and follow the instructions on this Enrollment Form
- Complete sections marked Step 1 and Step 2 on the Application
- Have your or your child’s doctor complete sections marked Step 3 and Step 4
- Submit proof of income, as instructed on the form, under PATIENT ASSISTANCE PROGRAM INSTRUCTIONS
- Fax page 1 of the form to 800-887-1338
Resources
Sickle Cell Disease Centers of Excellence
- Cincinnati Children's Sickle Cell Center
- The Center of Excellence in Sickle Cell Disease - Boston University
- The University of South Alabama Comprehensive Sickle Cell Center
- Sickle Cell Infusion Center for Adults
- Vanderbilt Center of Excellence in Sickle Cell Disease
- Harlem Hospital Center
- Southwestern Comprehensive Sickle Cell Center
- Northern California Comprehensive Sickle Cell Care Center
Sickle Cell Disease Associations and Advocacy Groups
INDICATION AND IMPORTANT SAFETY INFORMATION
IMPORTANT SAFETY INFORMATION
WARNING: LOW BLOOD CELL COUNT and CANCER
See Full Prescribing Information for complete Boxed Warning.Low blood cell counts are common with SIKLOS, including low red blood cells, white blood cells, and platelets, and can be severe and life threatening. If your white blood cell count becomes very low, you are at increased risk for infection. Your healthcare provider will check your blood cell counts before and every 2 weeks during treatment with SIKLOS. Your healthcare provider may change your dose or tell you to stop taking SIKLOS if you have low blood cell counts. Tell your healthcare provider right away if you get any of the following symptoms: fever or chills; shortness of breath; body aches; unusual headache; feeling very tired; bleeding or unexplained bruising.
Cancer. Some people have developed cancer, such as leukemia and skin cancer, after taking SIKLOS for a long time. Your healthcare provider will check you for cancer. You should protect your skin from the sun using sunblock, hats, and sun-protective clothing.
WHAT IS THE MOST IMPORTANT INFORMATION YOU SHOULD KNOW ABOUT SIKLOS?
- SIKLOS can harm your unborn baby.
- For females taking SIKLOS who can become pregnant:
- You should talk with your healthcare provider about the risks of SIKLOS to your unborn baby.
- You should use effective birth control during treatment with SIKLOS and for at least 6 months after treatment with SIKLOS.
- Your healthcare provider will perform a pregnancy test before you start treatment with SIKLOS. Tell your healthcare provider right away if you become pregnant or think you may be pregnant.
- For males taking SIKLOS: SIKLOS can affect your sperm. If you have a female sexual partner who can become pregnant, you should use effective birth control during treatment with SIKLOS and for at least 6 months after treatment.
- SIKLOS may cause fertility problems in males. Talk to your healthcare provider if this is a concern for you.
WHO SHOULD NOT TAKE SIKLOS
Do not take SIKLOS if you are allergic to hydroxyurea or any of the ingredients in SIKLOS. See the Medication Guide for a list of the ingredients in SIKLOS.
WHAT SHOULD YOU TELL YOUR HEALTH CARE PROVIDER BEFORE TAKING SIKLOS?
Tell your healthcare provider about all of your medical conditions, including if you:
- have kidney problems or are receiving hemodialysis
- have liver problems
- have human immunodeficiency virus (HIV) or take HIV medicines. Taking SIKLOS with certain HIV medicines can cause serious reactions and may lead to death.
- have increased levels of uric acid in your blood (hyperuricemia)
- have a history of receiving interferon therapy or are currently receiving interferon therapy
- plan to receive any vaccinations. You should not receive “live vaccines” during treatment with SIKLOS.
- are pregnant or plan to become pregnant. See "What is the most important information I should know about SIKLOS?"
- are breastfeeding or plan to breastfeed. It is not known if SIKLOS can pass into your breast milk. Do not breastfeed during treatment with SIKLOS.
- are using a continuous glucose monitor (CGM) to test your blood glucose. Talk to the healthcare provider that prescribed your CGM about whether it is safe to use while you are taking SIKLOS.
- Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
WHAT ARE THE POSSIBLE SIDE EFFECTS OF SIKLOS?
SIKLOS may cause serious side effects, including:
See "What is the most important information I should know about SIKLOS"
- Skin ulcers, including leg ulcers, and death of skin tissue (gangrene) have happened in people who take SIKLOS. This has happened most often in people who receive interferon therapy or have a history of interferon therapy. Your healthcare provider will decrease your dose or stop treatment with SIKLOS if you develop any skin ulcers.
- Enlarged red blood cells (macrocytosis). Macrocytosis is common in people who take SIKLOS and can make it difficult to detect a decrease of folic acid. Your healthcare provider may prescribe a folic acid supplement for you.
- Hemolytic Anemia, the fast breakdown of red blood cells, has happened in people who take SIKLOS. Tell your healthcare provider I you develop yellowing of your skin (jaundice) or blood in your urine. Your healthcare provider may do blood tests if you have persistent or worsening anemia not related to sickle cell anemia.
The most common side effects of SIKLOS in children include: infections and low white blood cells.
The most common side effects of SIKLOS in adults include: infections, headache, and dry skin.
These are not all the possible side effects of SIKLOS.
You are encouraged to report negative side effects of prescription drugs to the FDA at fda.gov/medwatch, or 1-800-FDA-1088.
Please read the Full Prescribing Information, including Boxed Warning, Medication Guide and Instructions for Use, at SIKLOSusa.com.
SIKLOS is a prescription medicine that is used to reduce the frequency of painful crises and reduce the need for blood transfusions in adults and children, 2 years of age and older, with sickle cell anemia with recurrent moderate to severe painful crises. It is not known if SIKLOS is safe and effective in children less than 2 years of age.
INDICATION AND IMPORTANT SAFETY INFORMATION
IMPORTANT SAFETY INFORMATION
WARNING: LOW BLOOD CELL COUNT and CANCER
See Full Prescribing Information for complete Boxed Warning.Low blood cell counts are common with SIKLOS, including low red blood cells, white blood cells, and platelets, and can be severe and life threatening. If your white blood cell count becomes very low, you are at increased risk for infection. Your healthcare provider will check your blood cell counts before and every 2 weeks during treatment with SIKLOS. Your healthcare provider may change your dose or tell you to stop taking SIKLOS if you have low blood cell counts. Tell your healthcare provider right away if you get any of the following symptoms: fever or chills; shortness of breath; body aches; unusual headache; feeling very tired; bleeding or unexplained bruising.
Cancer. Some people have developed cancer, such as leukemia and skin cancer, after taking SIKLOS for a long time. Your healthcare provider will check you for cancer. You should protect your skin from the sun using sunblock, hats, and sun-protective clothing.
SIKLOS is a prescription medicine that is used to reduce the frequency of painful crises and reduce the need for blood transfusions in adults and children, 2 years of age and older, with sickle cell anemia with recurrent moderate to severe painful crises. It is not known if SIKLOS is safe and effective in children less than 2 years of age.
