Treating Sickle Cell Disease (SCD)

What is Hydroxyurea?
Hydroxyurea (HU) is well established in the medical community as the "Gold Standard" treatment for sickle cell disease.
HU is a prescription medicine used to treat people with SCD since the 1980s (over 40 years!).
HU helps your red blood cells stay larger, rounder, and more flexible – making them less likely to turn into a sickle or curved shape.

Normal red blood cells are round and flexible and can move easily through blood vessels.
Importance of Early & Continued Treatment with Hydroxyurea
Hydroxyurea reduces the symptoms and complications that can arise from SCD. Treatment with HU helps reduce:

Vaso-Occlusive Episodes+Painful Crises

Blood Transfusions

Acute Chest Syndrome

Hospitalizations
It is important to treat symptoms of sickle cell disease as early as possible and to continue HU treatment your entire life to reduce the number of SCD events.
What is Siklos®?


- Dissolvability
- Flexible Dosing & administration
- Scored & Breakable tablets
- 100 mg & 1,000 mg Strengths
The FIRST and ONLY hydroxyurea-based treatment for patients 2 years and older with sickle cell anemia offering flexible dosing & administration.
Siklos® can be swallowed whole or dissolved in a small amount of water. No compounding is needed!
Siklos® is an FDA-approved prescription medication that reduces the frequency of painful crises and the need for blood transfusions in adults and children, 2 years of age and older, with sickle cell anemia with recurrent moderate to severe painful crises.
It is not known if Siklos® is safe and effective in children less than 2 years.

Start treatment early with Siklos® and continue into adulthood to get the best result from HU.
As an adult, you can still start on Siklos® and see benefits from HU treatment.
Why Siklos®?

HU treatment, your way!

Siklos® has been studied and proven safe and effective for adults and children 2 years of age and older.
- Scored & Breakable Tablets
- 100 mg & 1,000 mg Strengths
- Flexible Dosing & administration
- No compounding needed
Does your child struggle with taking compounded hydroxyurea (HU) medication? Insurance no longer covering compounded prescription medicines?
Siklos® is the ONLY dissolvable hydroxyurea medication.
Switch to Siklos® - no compounding needed!
Do you struggle with taking your HU capsules?
Siklos® is the ONLY hydroxyurea medication that comes in a 1,000 mg strength tablet.
Switch to Siklos® - take fewer HU pills daily!
Siklos® is the ONLY breakable/dissolvable hydroxyurea medication.
Switch to Siklos® - break the Siklos tablets into smaller pieces or dissolve in water.
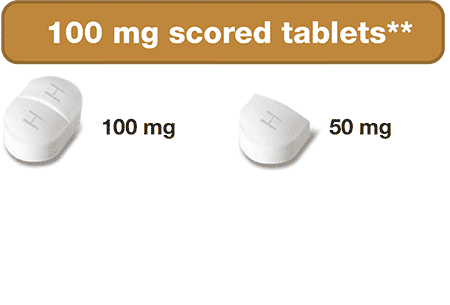
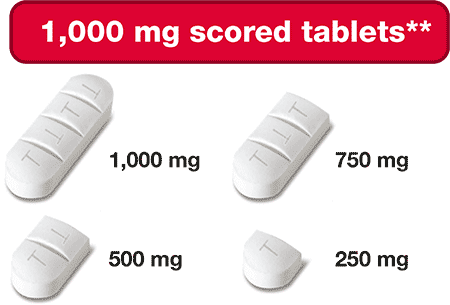
The ONLY hydroxyurea medication that can be taken 2 different ways

- Dissolve in a small amount of water in a teaspoon immediately before taking
- Swallow the tablet whole with a glass of water
- Scored tablets can be broken into smaller pieces if needed
It is important to take Siklos® once every day, at the same time each day, with a glass of water to get the most benefit from your hydroxyurea treatment.
Siklos® is the ONLY hydroxyurea that offers a choice on how you or your child can take Siklos®.
Dissolve Siklos® for your young child and as your child grows, he or she can take Siklos® by swallowing it whole. Siklos® can even be broken into smaller pieces to help with this.
View & print the Instructions for Taking Siklos®
Siklos® tablets must be handled with care. Follow applicable special handling and disposal procedures.
Do not take Siklos® if you are allergic to hydroxyurea or any of the ingredients in Siklos®.
How Siklos® Helps
Siklos® helps keep red blood cells round and flexible so they can travel more easily through blood vessels. This may reduce the painful episodes and some of the complications of sickle cell disease (known as SCD events).

Siklos® is proven to decrease how often complications occur with SCD for BOTH children and adults
Siklos® was proven safe & effective in the European Sickle Cell Disease Cohort Study (ESCORT-HU).
After 12 months of Siklos® treatment, the frequency and number of SCD events decreased for both pediatric and adult patients:
- Painful crises/vaso-occlusive episodes
- Acute Chest Syndrome
- Hospitalizations
- Blood transfusions


Siklos® Clinical Study – Proven Safe & Effective in Children Over 2 years & Adults
In the ESCORT-HU study, SCD patients were treated with Siklos ® and were studied for at least 12 months:
- Over 400 children from 2-18 years of age with SCD were included in the trial of which 141 patients were naive to HU treatment and analyzable after 12 months of Siklos® treatment
- 1,077 adult patients were included in the trial of which 436 patients were naive to HU treatment. There were 370 evaluable patients who had at least 12 months follow-up.
After 12 months of Siklos treatment, children ages 2 and older (n = 141):
- Had fewer painful crises/vaso-occlusive episodes (n=120)
- Number of patients with at least 1 episode of painful crises decreased by 26.5%
(From 83 patients (69%) with a least 1 episode of vaso-occlusive episode down to 51 patients (42.5%) with at least 1 episode)
- Number of patients with at least 1 episode of painful crises decreased by 26.5%
- Had fewer Acute Chest Syndrome episodes (a type of painful crises) (n=123)
- Number of patients with at least 1 episode of Acute Chest Syndrome decreased by 18%
(From 29 patients (24%) with a least 1 episode of ACS down to 7 patients (6%) with at least 1 episode)
- Number of patients with at least 1 episode of Acute Chest Syndrome decreased by 18%
- Had fewer hospitalizations (n=110)
- Number of patients needing to be hospitalized decreased by 33%
(From 83 patients (75%) needing to be hospitalized down to 46 patients (42%) needing to be hospitalized)
- Number of patients needing to be hospitalized decreased by 33%
- Needed fewer blood transfusions (n=122)
- Number of patients needing blood transfusions decreased by 23%
(From 56 patients (46%) needing blood transfusions down to 28 patients (23%) needing blood transfusions)
- Number of patients needing blood transfusions decreased by 23%

Vaso-Occlusive Episodes+Painful Crises
Decreased by 26.5%

Hospitalizations
Decreased by 33%

Acute Chest Syndrome
Decreased by 18%

Blood Transfusions
Decreased by 23%
After 12 months of Siklos treatment, adult patients (n = 370):
- Had fewer painful crises/vaso-occlusive episodes (n=367)
- Number of patients with at least 1 episode of painful crises decreased by 25.4%
(From 234 patients (63.8%) with a least 1 episode of vaso-occlusive episode down to 141 patients (38.4%) with at least 1 episode)
- Number of patients with at least 1 episode of painful crises decreased by 25.4%
- Had fewer Acute Chest Syndrome episodes (a type of painful crises) (n=364)
- Number of patients with at least 1 episode of Acute Chest Syndrome decreased by 17.8%
(From 92 patients (25.2%) with a least 1 episode of ACS down to 27 patients (7.4%) with at least 1 episode)
- Number of patients with at least 1 episode of Acute Chest Syndrome decreased by 17.8%
- Had fewer hospitalizations (n=366)
- Number of patients needing to be hospitalized decreased by 27.4%
(From 214 patients (58.5%) needing to be hospitalized down to 114 patients (31.1%) needing to be hospitalized)
- Number of patients needing to be hospitalized decreased by 27.4%
- Needed fewer blood transfusions (n=365)
- Number of patients needing blood transfusions decreased by 24.4%
(From 158 patients (43.3%) needing blood transfusions down to 69 patients (18.9%) needing blood transfusions)
- Number of patients needing blood transfusions decreased by 24.4%

Vaso-Occlusive Episodes+Painful Crises
Decreased by 25.4%

Hospitalizations
Decreased by 17.8%

Acute Chest Syndrome
Decreased by 27.4%

Blood Transfusions
Decreased by 24.4%
Side Effects of Siklos®
WARNING: LOW BLOOD CELL COUNT and CANCER
See Full Prescribing Information for complete Boxed Warning.Low blood cell counts are common with SIKLOS, including low red blood cells, white blood cells, and platelets, and can be severe and life threatening. If your white blood cell count becomes very low, you are at increased risk for infection. Your healthcare provider will check your blood cell counts before and every 2 weeks during treatment with SIKLOS. Your healthcare provider may change your dose or tell you to stop taking SIKLOS if you have low blood cell counts. Tell your healthcare provider right away if you get any of the following symptoms: fever or chills; shortness of breath; body aches; unusual headache; feeling very tired; bleeding or unexplained bruising.
Cancer. Some people have developed cancer, such as leukemia and skin cancer, after taking SIKLOS for a long time. Your healthcare provider will check you for cancer. You should protect your skin from the sun using sunblock, hats, and sun-protective clothing.
Siklos® may cause serious side effects, including:
- Skin ulcers, including leg ulcers, and death of skin tissue (gangrene) have happened in people who take SIKLOS. This has happened most often in people who receive interferon therapy or have a history of interferon therapy. Your healthcare provider will decrease your dose or stop treatment with SIKLOS if you develop any skin ulcers.
- Enlarged red blood cells (macrocytosis). Macrocytosis is common in people who take SIKLOS and can make it difficult to detect a decrease of folic acid. Your healthcare provider may prescribe a folic acid supplement for you.
- Hemolytic Anemia, the fast breakdown of red blood cells, has happened in people who take SIKLOS. Tell your healthcare provider if you develop yellowing of your skin (jaundice) or blood in your urine. Your healthcare provider may do blood tests if you have persistent or worsening anemia not related to sickle cell anemia.
The most common side effects of Siklos ® in children include: infections and low white blood cells.
The most common side effects of Siklos ® in adults include: infections, headache, and dry skin.
These are not all the possible side effects of Siklos®.
You are encouraged to report negative side effects of prescription drugs to the FDA at www.fda.gov/medwatch, or 1-800-FDA-1088.
INDICATION AND IMPORTANT SAFETY INFORMATION
IMPORTANT SAFETY INFORMATION
WARNING: LOW BLOOD CELL COUNT and CANCER
See Full Prescribing Information for complete Boxed Warning.Low blood cell counts are common with SIKLOS, including low red blood cells, white blood cells, and platelets, and can be severe and life threatening. If your white blood cell count becomes very low, you are at increased risk for infection. Your healthcare provider will check your blood cell counts before and every 2 weeks during treatment with SIKLOS. Your healthcare provider may change your dose or tell you to stop taking SIKLOS if you have low blood cell counts. Tell your healthcare provider right away if you get any of the following symptoms: fever or chills; shortness of breath; body aches; unusual headache; feeling very tired; bleeding or unexplained bruising.
Cancer. Some people have developed cancer, such as leukemia and skin cancer, after taking SIKLOS for a long time. Your healthcare provider will check you for cancer. You should protect your skin from the sun using sunblock, hats, and sun-protective clothing.
WHAT IS THE MOST IMPORTANT INFORMATION YOU SHOULD KNOW ABOUT SIKLOS?
- SIKLOS can harm your unborn baby.
- For females taking SIKLOS who can become pregnant:
- You should talk with your healthcare provider about the risks of SIKLOS to your unborn baby.
- You should use effective birth control during treatment with SIKLOS and for at least 6 months after treatment with SIKLOS.
- Your healthcare provider will perform a pregnancy test before you start treatment with SIKLOS. Tell your healthcare provider right away if you become pregnant or think you may be pregnant.
- For males taking SIKLOS: SIKLOS can affect your sperm. If you have a female sexual partner who can become pregnant, you should use effective birth control during treatment with SIKLOS and for at least 6 months after treatment.
- SIKLOS may cause fertility problems in males. Talk to your healthcare provider if this is a concern for you.
WHO SHOULD NOT TAKE SIKLOS
Do not take SIKLOS if you are allergic to hydroxyurea or any of the ingredients in SIKLOS. See the Medication Guide for a list of the ingredients in SIKLOS.
WHAT SHOULD YOU TELL YOUR HEALTH CARE PROVIDER BEFORE TAKING SIKLOS?
Tell your healthcare provider about all of your medical conditions, including if you:
- have kidney problems or are receiving hemodialysis
- have liver problems
- have human immunodeficiency virus (HIV) or take HIV medicines. Taking SIKLOS with certain HIV medicines can cause serious reactions and may lead to death.
- have increased levels of uric acid in your blood (hyperuricemia)
- have a history of receiving interferon therapy or are currently receiving interferon therapy
- plan to receive any vaccinations. You should not receive “live vaccines” during treatment with SIKLOS.
- are pregnant or plan to become pregnant. See "What is the most important information I should know about SIKLOS?"
- are breastfeeding or plan to breastfeed. It is not known if SIKLOS can pass into your breast milk. Do not breastfeed during treatment with SIKLOS.
- are using a continuous glucose monitor (CGM) to test your blood glucose. Talk to the healthcare provider that prescribed your CGM about whether it is safe to use while you are taking SIKLOS.
- Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
WHAT ARE THE POSSIBLE SIDE EFFECTS OF SIKLOS?
SIKLOS may cause serious side effects, including:
See "What is the most important information I should know about SIKLOS"
- Skin ulcers, including leg ulcers, and death of skin tissue (gangrene) have happened in people who take SIKLOS. This has happened most often in people who receive interferon therapy or have a history of interferon therapy. Your healthcare provider will decrease your dose or stop treatment with SIKLOS if you develop any skin ulcers.
- Enlarged red blood cells (macrocytosis). Macrocytosis is common in people who take SIKLOS and can make it difficult to detect a decrease of folic acid. Your healthcare provider may prescribe a folic acid supplement for you.
- Hemolytic Anemia, the fast breakdown of red blood cells, has happened in people who take SIKLOS. Tell your healthcare provider I you develop yellowing of your skin (jaundice) or blood in your urine. Your healthcare provider may do blood tests if you have persistent or worsening anemia not related to sickle cell anemia.
The most common side effects of SIKLOS in children include: infections and low white blood cells.
The most common side effects of SIKLOS in adults include: infections, headache, and dry skin.
These are not all the possible side effects of SIKLOS.
You are encouraged to report negative side effects of prescription drugs to the FDA at fda.gov/medwatch, or 1-800-FDA-1088.
Please read the Full Prescribing Information, including Boxed Warning, Medication Guide and Instructions for Use, at SIKLOSusa.com.
SIKLOS is a prescription medicine that is used to reduce the frequency of painful crises and reduce the need for blood transfusions in adults and children, 2 years of age and older, with sickle cell anemia with recurrent moderate to severe painful crises. It is not known if SIKLOS is safe and effective in children less than 2 years of age.
INDICATION AND IMPORTANT SAFETY INFORMATION
IMPORTANT SAFETY INFORMATION
WARNING: LOW BLOOD CELL COUNT and CANCER
See Full Prescribing Information for complete Boxed Warning.Low blood cell counts are common with SIKLOS, including low red blood cells, white blood cells, and platelets, and can be severe and life threatening. If your white blood cell count becomes very low, you are at increased risk for infection. Your healthcare provider will check your blood cell counts before and every 2 weeks during treatment with SIKLOS. Your healthcare provider may change your dose or tell you to stop taking SIKLOS if you have low blood cell counts. Tell your healthcare provider right away if you get any of the following symptoms: fever or chills; shortness of breath; body aches; unusual headache; feeling very tired; bleeding or unexplained bruising.
Cancer. Some people have developed cancer, such as leukemia and skin cancer, after taking SIKLOS for a long time. Your healthcare provider will check you for cancer. You should protect your skin from the sun using sunblock, hats, and sun-protective clothing.
WHAT IS THE MOST IMPORTANT INFORMATION YOU SHOULD KNOW ABOUT SIKLOS?
- SIKLOS can harm your unborn baby.
- For females taking SIKLOS who can become pregnant:
- You should talk with your healthcare provider about the risks of SIKLOS to your unborn baby.
- You should use effective birth control during treatment with SIKLOS and for at least 6 months after treatment with SIKLOS.
- Your healthcare provider will perform a pregnancy test before you start treatment with SIKLOS. Tell your healthcare provider right away if you become pregnant or think you may be pregnant.
- For males taking SIKLOS: SIKLOS can affect your sperm. If you have a female sexual partner who can become pregnant, you should use effective birth control during treatment with SIKLOS and for at least 6 months after treatment.
- SIKLOS may cause fertility problems in males. Talk to your healthcare provider if this is a concern for you.
WHO SHOULD NOT TAKE SIKLOS
Do not take SIKLOS if you are allergic to hydroxyurea or any of the ingredients in SIKLOS. See the Medication Guide for a list of the ingredients in SIKLOS.
WHAT SHOULD YOU TELL YOUR HEALTH CARE PROVIDER BEFORE TAKING SIKLOS?
Tell your healthcare provider about all of your medical conditions, including if you:
- have kidney problems or are receiving hemodialysis
- have liver problems
- have human immunodeficiency virus (HIV) or take HIV medicines. Taking SIKLOS with certain HIV medicines can cause serious reactions and may lead to death.
- have increased levels of uric acid in your blood (hyperuricemia)
- have a history of receiving interferon therapy or are currently receiving interferon therapy
- plan to receive any vaccinations. You should not receive “live vaccines” during treatment with SIKLOS.
- are pregnant or plan to become pregnant. See "What is the most important information I should know about SIKLOS?"
- are breastfeeding or plan to breastfeed. It is not known if SIKLOS can pass into your breast milk. Do not breastfeed during treatment with SIKLOS.
- are using a continuous glucose monitor (CGM) to test your blood glucose. Talk to the healthcare provider that prescribed your CGM about whether it is safe to use while you are taking SIKLOS.
- Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
WHAT ARE THE POSSIBLE SIDE EFFECTS OF SIKLOS?
SIKLOS may cause serious side effects, including:
See "What is the most important information I should know about SIKLOS"
- Skin ulcers, including leg ulcers, and death of skin tissue (gangrene) have happened in people who take SIKLOS. This has happened most often in people who receive interferon therapy or have a history of interferon therapy. Your healthcare provider will decrease your dose or stop treatment with SIKLOS if you develop any skin ulcers.
- Enlarged red blood cells (macrocytosis). Macrocytosis is common in people who take SIKLOS and can make it difficult to detect a decrease of folic acid. Your healthcare provider may prescribe a folic acid supplement for you.
- Hemolytic Anemia, the fast breakdown of red blood cells, has happened in people who take SIKLOS. Tell your healthcare provider I you develop yellowing of your skin (jaundice) or blood in your urine. Your healthcare provider may do blood tests if you have persistent or worsening anemia not related to sickle cell anemia.
The most common side effects of SIKLOS in children include: infections and low white blood cells.
The most common side effects of SIKLOS in adults include: infections, headache, and dry skin.
These are not all the possible side effects of SIKLOS.
You are encouraged to report negative side effects of prescription drugs to the FDA at fda.gov/medwatch, or 1-800-FDA-1088.
Please read the Full Prescribing Information, including Boxed Warning, Medication Guide and Instructions for Use, at SIKLOSusa.com.
SIKLOS is a prescription medicine that is used to reduce the frequency of painful crises and reduce the need for blood transfusions in adults and children, 2 years of age and older, with sickle cell anemia with recurrent moderate to severe painful crises. It is not known if SIKLOS is safe and effective in children less than 2 years of age.
INDICATION AND IMPORTANT SAFETY INFORMATION
IMPORTANT SAFETY INFORMATION
WARNING: LOW BLOOD CELL COUNT and CANCER
See Full Prescribing Information for complete Boxed Warning.Low blood cell counts are common with SIKLOS, including low red blood cells, white blood cells, and platelets, and can be severe and life threatening. If your white blood cell count becomes very low, you are at increased risk for infection. Your healthcare provider will check your blood cell counts before and every 2 weeks during treatment with SIKLOS. Your healthcare provider may change your dose or tell you to stop taking SIKLOS if you have low blood cell counts. Tell your healthcare provider right away if you get any of the following symptoms: fever or chills; shortness of breath; body aches; unusual headache; feeling very tired; bleeding or unexplained bruising.
Cancer. Some people have developed cancer, such as leukemia and skin cancer, after taking SIKLOS for a long time. Your healthcare provider will check you for cancer. You should protect your skin from the sun using sunblock, hats, and sun-protective clothing.
SIKLOS is a prescription medicine that is used to reduce the frequency of painful crises and reduce the need for blood transfusions in adults and children, 2 years of age and older, with sickle cell anemia with recurrent moderate to severe painful crises. It is not known if SIKLOS is safe and effective in children less than 2 years of age.
