Taking Siklos®
HU treatment, your way!
2 ways Siklos® can be taken
Siklos® is the ONLY hydroxyurea treatment that can be taken 2 different ways:
- Dissolve in a small amount of water in a teaspoon immediately before taking. The tablet dissolves within about 1 minute.
- Swallow the tablet whole with a glass of water
- Scored tablets can be broken into smaller pieces if needed
It is important to take Siklos® once every day, at the same time each day, with a glass of water to get the most benefit from your hydroxyurea treatment.
Siklos® offers a choice on how you or your child can take Siklos®
Dissolve Siklos® for your young child and as your child grows, he or she can take Siklos® by swallowing it whole. Siklos® can even be broken into smaller pieces to help with this.
Siklos® tablets must be handled with care. Follow applicable special handling and disposal procedures as detailed in the below Dissolving Instructions and Handling & Breaking Instructions.
View and print:
Instructions for Taking Siklos®:
Ver e imprimir el:
Instrucciones para tomar Siklos®:
Siklos® is available in:
- 100 mg scored tablets – can be split into two 50 mg portions
- 1,000 mg triple-scored - can be split into four 250 mg portions
These dosing options allow for more precise dose adjustments based on your or your child’s body weight and clinical response. This can be helpful when your child is growing and weight keeps changing!
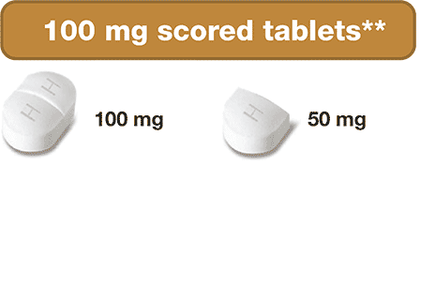
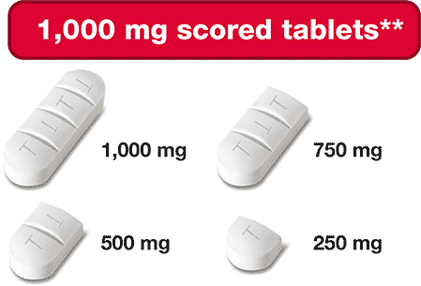
If too much Siklos® is taken, call your doctor or go to the nearest hospital emergency room right away.

Importance of Regular Doctor Visits during Treatment with Siklos®
Regular doctor visits while on Siklos® are very important to ensure that the treatment is working as expected and to check for any potential side effects.
Your doctor will check your blood cell counts before and every 2 weeks during treatment with Siklos®. Your doctor may change your dose or tell you to stop temporarily taking Siklos® if you have low blood cell counts.
Tell your doctor right away if you get any of the following symptoms:
- Fever or chills
- Body aches
- Feeling very tired
- Shortness of breath
- Unusual headache
- Bleeding or unexplained bruising

While taking Siklos®, your doctor will:
- Make dose adjustments as weight changes
- Monitor blood cell counts every 2 weeks
- Monitor growth in children because sickle cell disease can affect growth and development
The monitoring of blood cell counts every 2 weeks is important to ensure you or your child is on the appropriate dose:
- Low blood cell counts are common with Siklos®, including low red blood cells, white blood cells, and platelets, and can be severe and life-threatening.
- Children aged 2-16 years have a higher risk of low blood cell count than patients that are more than 16 years of age

Hydroxyurea may take time to work. Don’t be discouraged! It may take time for your doctor to determine the appropriate dose for you or your child.


INDICATION AND IMPORTANT SAFETY INFORMATION
IMPORTANT SAFETY INFORMATION
WARNING: LOW BLOOD CELL COUNT and CANCER
See Full Prescribing Information for complete Boxed Warning.Low blood cell counts are common with SIKLOS, including low red blood cells, white blood cells, and platelets, and can be severe and life threatening. If your white blood cell count becomes very low, you are at increased risk for infection. Your healthcare provider will check your blood cell counts before and every 2 weeks during treatment with SIKLOS. Your healthcare provider may change your dose or tell you to stop taking SIKLOS if you have low blood cell counts. Tell your healthcare provider right away if you get any of the following symptoms: fever or chills; shortness of breath; body aches; unusual headache; feeling very tired; bleeding or unexplained bruising.
Cancer. Some people have developed cancer, such as leukemia and skin cancer, after taking SIKLOS for a long time. Your healthcare provider will check you for cancer. You should protect your skin from the sun using sunblock, hats, and sun-protective clothing.
WHAT IS THE MOST IMPORTANT INFORMATION YOU SHOULD KNOW ABOUT SIKLOS?
- SIKLOS can harm your unborn baby.
- For females taking SIKLOS who can become pregnant:
- You should talk with your healthcare provider about the risks of SIKLOS to your unborn baby.
- You should use effective birth control during treatment with SIKLOS and for at least 6 months after treatment with SIKLOS.
- Your healthcare provider will perform a pregnancy test before you start treatment with SIKLOS. Tell your healthcare provider right away if you become pregnant or think you may be pregnant.
- For males taking SIKLOS: SIKLOS can affect your sperm. If you have a female sexual partner who can become pregnant, you should use effective birth control during treatment with SIKLOS and for at least 6 months after treatment.
- SIKLOS may cause fertility problems in males. Talk to your healthcare provider if this is a concern for you.
WHO SHOULD NOT TAKE SIKLOS
Do not take SIKLOS if you are allergic to hydroxyurea or any of the ingredients in SIKLOS. See the Medication Guide for a list of the ingredients in SIKLOS.
WHAT SHOULD YOU TELL YOUR HEALTH CARE PROVIDER BEFORE TAKING SIKLOS?
Tell your healthcare provider about all of your medical conditions, including if you:
- have kidney problems or are receiving hemodialysis
- have liver problems
- have human immunodeficiency virus (HIV) or take HIV medicines. Taking SIKLOS with certain HIV medicines can cause serious reactions and may lead to death.
- have increased levels of uric acid in your blood (hyperuricemia)
- have a history of receiving interferon therapy or are currently receiving interferon therapy
- plan to receive any vaccinations. You should not receive “live vaccines” during treatment with SIKLOS.
- are pregnant or plan to become pregnant. See "What is the most important information I should know about SIKLOS?"
- are breastfeeding or plan to breastfeed. It is not known if SIKLOS can pass into your breast milk. Do not breastfeed during treatment with SIKLOS.
- are using a continuous glucose monitor (CGM) to test your blood glucose. Talk to the healthcare provider that prescribed your CGM about whether it is safe to use while you are taking SIKLOS.
- Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
WHAT ARE THE POSSIBLE SIDE EFFECTS OF SIKLOS?
SIKLOS may cause serious side effects, including:
See "What is the most important information I should know about SIKLOS"
- Skin ulcers, including leg ulcers, and death of skin tissue (gangrene) have happened in people who take SIKLOS. This has happened most often in people who receive interferon therapy or have a history of interferon therapy. Your healthcare provider will decrease your dose or stop treatment with SIKLOS if you develop any skin ulcers.
- Enlarged red blood cells (macrocytosis). Macrocytosis is common in people who take SIKLOS and can make it difficult to detect a decrease of folic acid. Your healthcare provider may prescribe a folic acid supplement for you.
- Hemolytic Anemia, the fast breakdown of red blood cells, has happened in people who take SIKLOS. Tell your healthcare provider I you develop yellowing of your skin (jaundice) or blood in your urine. Your healthcare provider may do blood tests if you have persistent or worsening anemia not related to sickle cell anemia.
The most common side effects of SIKLOS in children include: infections and low white blood cells.
The most common side effects of SIKLOS in adults include: infections, headache, and dry skin.
These are not all the possible side effects of SIKLOS.
You are encouraged to report negative side effects of prescription drugs to the FDA at fda.gov/medwatch, or 1-800-FDA-1088.
Please read the Full Prescribing Information, including Boxed Warning, Medication Guide and Instructions for Use, at SIKLOSusa.com.
SIKLOS is a prescription medicine that is used to reduce the frequency of painful crises and reduce the need for blood transfusions in adults and children, 2 years of age and older, with sickle cell anemia with recurrent moderate to severe painful crises. It is not known if SIKLOS is safe and effective in children less than 2 years of age.
INDICATION AND IMPORTANT SAFETY INFORMATION
IMPORTANT SAFETY INFORMATION
WARNING: LOW BLOOD CELL COUNT and CANCER
See Full Prescribing Information for complete Boxed Warning.Low blood cell counts are common with SIKLOS, including low red blood cells, white blood cells, and platelets, and can be severe and life threatening. If your white blood cell count becomes very low, you are at increased risk for infection. Your healthcare provider will check your blood cell counts before and every 2 weeks during treatment with SIKLOS. Your healthcare provider may change your dose or tell you to stop taking SIKLOS if you have low blood cell counts. Tell your healthcare provider right away if you get any of the following symptoms: fever or chills; shortness of breath; body aches; unusual headache; feeling very tired; bleeding or unexplained bruising.
Cancer. Some people have developed cancer, such as leukemia and skin cancer, after taking SIKLOS for a long time. Your healthcare provider will check you for cancer. You should protect your skin from the sun using sunblock, hats, and sun-protective clothing.
WHAT IS THE MOST IMPORTANT INFORMATION YOU SHOULD KNOW ABOUT SIKLOS?
- SIKLOS can harm your unborn baby.
- For females taking SIKLOS who can become pregnant:
- You should talk with your healthcare provider about the risks of SIKLOS to your unborn baby.
- You should use effective birth control during treatment with SIKLOS and for at least 6 months after treatment with SIKLOS.
- Your healthcare provider will perform a pregnancy test before you start treatment with SIKLOS. Tell your healthcare provider right away if you become pregnant or think you may be pregnant.
- For males taking SIKLOS: SIKLOS can affect your sperm. If you have a female sexual partner who can become pregnant, you should use effective birth control during treatment with SIKLOS and for at least 6 months after treatment.
- SIKLOS may cause fertility problems in males. Talk to your healthcare provider if this is a concern for you.
WHO SHOULD NOT TAKE SIKLOS
Do not take SIKLOS if you are allergic to hydroxyurea or any of the ingredients in SIKLOS. See the Medication Guide for a list of the ingredients in SIKLOS.
WHAT SHOULD YOU TELL YOUR HEALTH CARE PROVIDER BEFORE TAKING SIKLOS?
Tell your healthcare provider about all of your medical conditions, including if you:
- have kidney problems or are receiving hemodialysis
- have liver problems
- have human immunodeficiency virus (HIV) or take HIV medicines. Taking SIKLOS with certain HIV medicines can cause serious reactions and may lead to death.
- have increased levels of uric acid in your blood (hyperuricemia)
- have a history of receiving interferon therapy or are currently receiving interferon therapy
- plan to receive any vaccinations. You should not receive “live vaccines” during treatment with SIKLOS.
- are pregnant or plan to become pregnant. See "What is the most important information I should know about SIKLOS?"
- are breastfeeding or plan to breastfeed. It is not known if SIKLOS can pass into your breast milk. Do not breastfeed during treatment with SIKLOS.
- are using a continuous glucose monitor (CGM) to test your blood glucose. Talk to the healthcare provider that prescribed your CGM about whether it is safe to use while you are taking SIKLOS.
- Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
WHAT ARE THE POSSIBLE SIDE EFFECTS OF SIKLOS?
SIKLOS may cause serious side effects, including:
See "What is the most important information I should know about SIKLOS"
- Skin ulcers, including leg ulcers, and death of skin tissue (gangrene) have happened in people who take SIKLOS. This has happened most often in people who receive interferon therapy or have a history of interferon therapy. Your healthcare provider will decrease your dose or stop treatment with SIKLOS if you develop any skin ulcers.
- Enlarged red blood cells (macrocytosis). Macrocytosis is common in people who take SIKLOS and can make it difficult to detect a decrease of folic acid. Your healthcare provider may prescribe a folic acid supplement for you.
- Hemolytic Anemia, the fast breakdown of red blood cells, has happened in people who take SIKLOS. Tell your healthcare provider I you develop yellowing of your skin (jaundice) or blood in your urine. Your healthcare provider may do blood tests if you have persistent or worsening anemia not related to sickle cell anemia.
The most common side effects of SIKLOS in children include: infections and low white blood cells.
The most common side effects of SIKLOS in adults include: infections, headache, and dry skin.
These are not all the possible side effects of SIKLOS.
You are encouraged to report negative side effects of prescription drugs to the FDA at fda.gov/medwatch, or 1-800-FDA-1088.
Please read the Full Prescribing Information, including Boxed Warning, Medication Guide and Instructions for Use, at SIKLOSusa.com.
SIKLOS is a prescription medicine that is used to reduce the frequency of painful crises and reduce the need for blood transfusions in adults and children, 2 years of age and older, with sickle cell anemia with recurrent moderate to severe painful crises. It is not known if SIKLOS is safe and effective in children less than 2 years of age.
INDICATION AND IMPORTANT SAFETY INFORMATION
IMPORTANT SAFETY INFORMATION
WARNING: LOW BLOOD CELL COUNT and CANCER
See Full Prescribing Information for complete Boxed Warning.Low blood cell counts are common with SIKLOS, including low red blood cells, white blood cells, and platelets, and can be severe and life threatening. If your white blood cell count becomes very low, you are at increased risk for infection. Your healthcare provider will check your blood cell counts before and every 2 weeks during treatment with SIKLOS. Your healthcare provider may change your dose or tell you to stop taking SIKLOS if you have low blood cell counts. Tell your healthcare provider right away if you get any of the following symptoms: fever or chills; shortness of breath; body aches; unusual headache; feeling very tired; bleeding or unexplained bruising.
Cancer. Some people have developed cancer, such as leukemia and skin cancer, after taking SIKLOS for a long time. Your healthcare provider will check you for cancer. You should protect your skin from the sun using sunblock, hats, and sun-protective clothing.
SIKLOS is a prescription medicine that is used to reduce the frequency of painful crises and reduce the need for blood transfusions in adults and children, 2 years of age and older, with sickle cell anemia with recurrent moderate to severe painful crises. It is not known if SIKLOS is safe and effective in children less than 2 years of age.